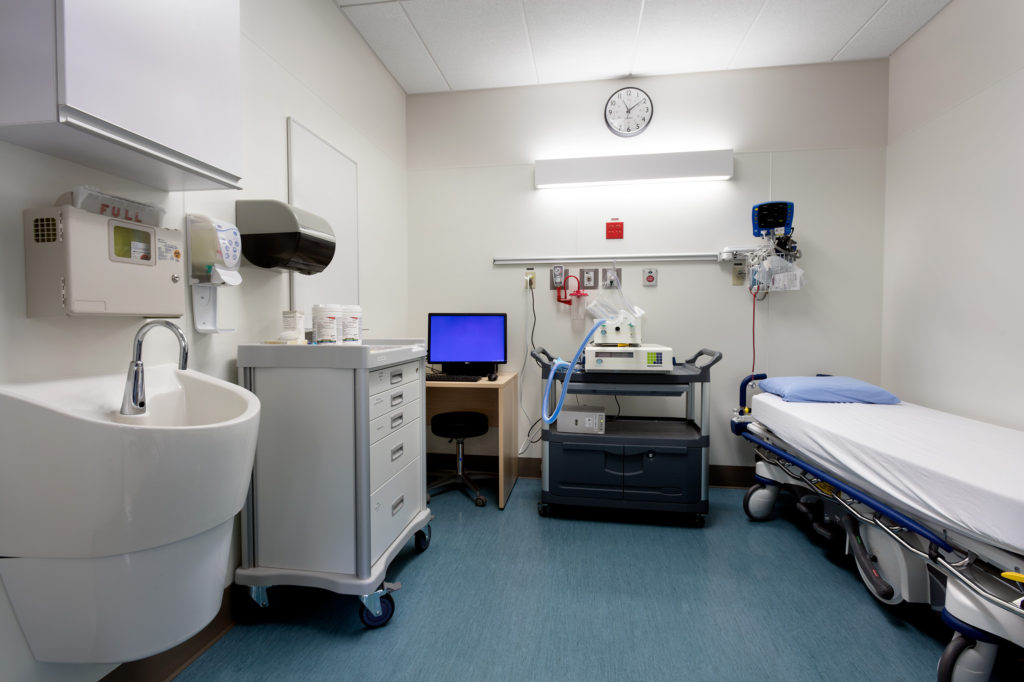
Clinical Investigation Unit
Opened in 2005, the Phase 1 unit is among the few Phase 1 units in Canada located within a major tertiary care facility: in this case, the University Hospital. The 3,300-square-foot, eight-bed ward is designed to assess drug safety, pharmacokinetics, pharmacodynamics, and tolerability in a controlled environment. You can conduct Phase 1–4 studies on your own or use our staff with their full range of clinical investigations experience.
Location
Unit 2C3, 2nd Floor
Walter C. MacKenzie Health Sciences Centre
Hours
7:00AM–3:15PM
Monday–Friday
Arrangements can be made for overnight or weekend studies.
Accommodations
- 8 beds (two four-bed rooms) with outpatient and inpatient flexibility
- All beds can be converted to inpatient beds for overnight and multi-day studies
- Bathroom with shower
- Rentable TV for each bed
- WiFi
- Private consultation room
Equipment
- Each bed has Philips monitors (BP, P, O2 sats, ECG), wall-mounted digital timer, Alaris infusion pump, oxygen, and suction
- Medifusion 3500 syringe pumps, calibrated electronic scales, tympanic thermometers, manual blood pressure cuff, and ophthalmoscope
- Preventive maintenance on all equipment done as per manufacturer specs by U of A Clinical Engineering Department. Maintenance records available upon request
Hospital Lab Services
- Immediate specimen transport to hospital core lab and clinical trials lab for processing
- Spot and serial blood collection via macro collection or intravenous catheter
- PICC line and IVAD certified
Kitchen
- Food service available through hospital dietary department
- Accommodates special diets
- Can accommodate food interaction and specialty diet studies
Unit CIU Lab
- Basic blood and body fluid processing facilities available
- Refrigerated Beckman centrifuge
- Two -70ºC remotely monitored freezers
- Class 2 Biologic Safety Hood
- Ice machine


CIU Staff
- Experienced in all aspects of clinical trials and diagnostic testing
- Good Clinical Practice (GCP) certification
- Chemotherapy certification
- IV starts for infusions and serial blood collection
- Macro collection for blood samples
- Create custom worksheets based on protocol requirements
- Basic blood processing
Safety
- Located within major tertiary hospital
- MET team and code team can respond to CIU
- Reaction medication immediately available
- Alarmed doors
- Double-locked medicine cabinet
Level 2 Containment
- Level 2 virus compliant for storage, reconstitution, and administration
- Full level 2 standard operating procedures
Pediatric Clinical Investigation Unit
The Pediatric Clinical Investigation Unit in the Stollery Children’s Hospital is an ideal research space where investigators can complete procedures, gather patient histories, and carry out physical exams. The space is staffed by RNs, is equipped with three curtained treatment spaces, and has an enclosed private room. This brand-new facility opened in 2014 and is conveniently located next door to the Stollery Pediatric Clinics.
Equipment
The Pediatric CIU offers investigators state-of-the-art monitoring equipment and specialty technology like the following.
- Computer on wheels
- Observation room stretcher
- Lab with centrifuge, microscopes, temperature-controlled freezer and fridge, biological safety hood
- Double-locked cabinet for samples, charts, and medications
- Exhaled nitric oxide testing
- Pea Pod Infant Body Composition System
Location
Ambulatory 2E, 2nd Floor
Oilers Clinic at Stollery Children’s Hospital
Hours
8:00AM–4:00PM
Monday–Friday
Arrangements can be made for overnight or weekend studies.